Zerit (Stavudine) vs Other HIV NRTIs Comparison Tool
About Stavudine (Zerit)
Zerit (stavudine) is an older NRTI approved in the mid-1990s. While effective, it's associated with significant mitochondrial toxicity including peripheral neuropathy and lipodystrophy. It's now recommended primarily in resource-limited settings due to cost considerations.
Key Characteristics
- Dosed as 30mg twice daily
- Used in combination therapy
- Can cause mitochondrial toxicity
- Less convenient dosing schedule
Major Side Effects
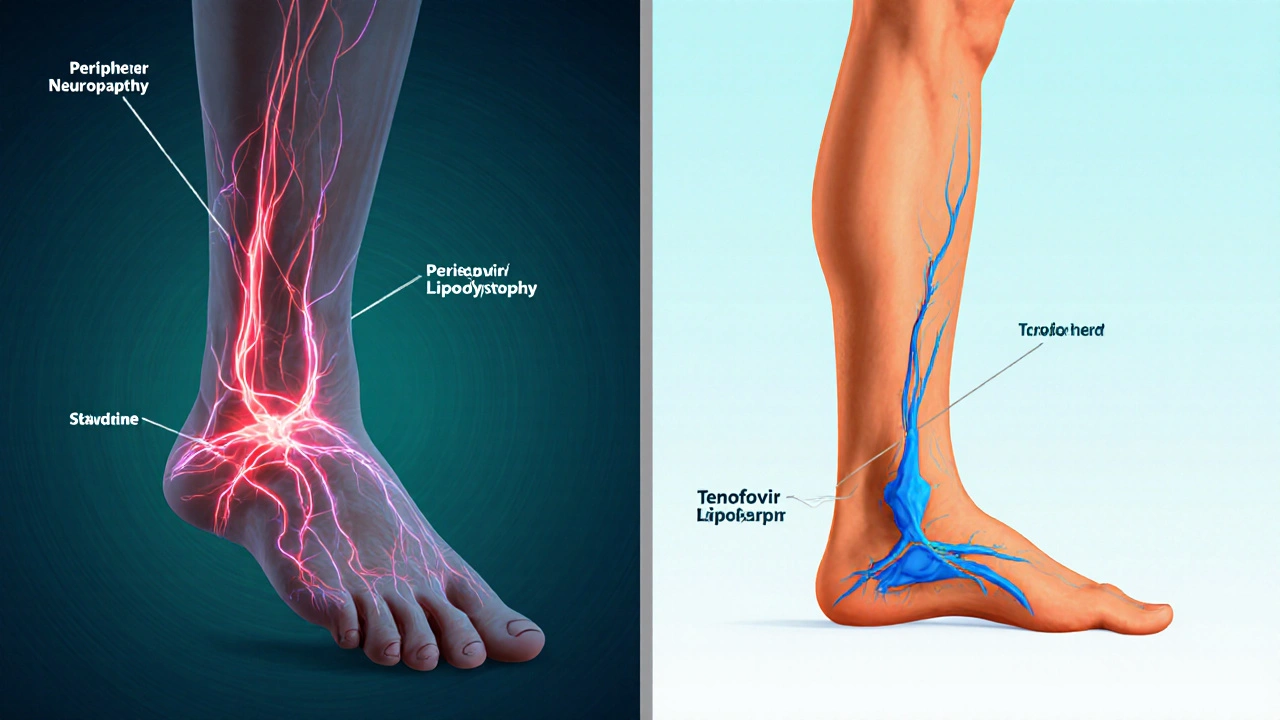
- Peripheral neuropathy (up to 20%)
- Lipodystrophy
- Hyperlactatemia
- Gastrointestinal upset
Key Takeaways
- Zerit (stavudine) is an older NRTI with higher toxicity than most modern alternatives.
- Tenofovir and emtricitabine are generally favored for their safety and once‑daily dosing.
- Cost and availability still make Zerit a viable option in low‑resource settings.
- Switching drugs requires careful monitoring of viral load and resistance patterns.
- Choose an NRTI based on efficacy, side‑effect profile, dosing convenience, and individual health status.
If you or someone you care for is on Zerit and wondering whether there’s a better option, you’re not alone. Patients and clinicians often grapple with questions like: Does a newer pill work faster? Will it cause fewer side effects? How much will it cost? This article breaks down the chemistry, the clinical data, and the real‑world factors that matter when you compare Zerit with its modern counterparts.
First, let’s define the main player.
When treating HIV, Zerit is the brand name for stavudine, a nucleoside reverse transcriptase inhibitor (NRTI) that blocks the HIV enzyme reverse transcriptase. It was first approved in the mid‑1990s and has been used in combination regimens worldwide.
Stavudine belongs to the Nucleoside Reverse Transcriptase Inhibitors (NRTIs), a class of drugs that mimic natural nucleosides and get incorporated into viral DNA, causing premature chain termination.
How Zerit Works and What It Looks Like in Practice
Stavudine is taken orally, usually 30mg twice daily for adults, though doses may be reduced for patients under 60kg or with renal impairment. The drug achieves plasma concentrations that suppress viral replication when combined with at least two other antiretrovirals, forming a complete antiretroviral therapy (ART) regimen.
Because it competes with natural thymidine, stavudine can interfere with mitochondrial DNA polymerase γ, leading to the notorious “mitochondrial toxicity” that shows up as peripheral neuropathy, lipodystrophy, and lipoatrophy.
Side‑Effect Profile - Why Zerit Fell Out of Favor
The most common adverse events reported in clinical trials include:
- Peripheral neuropathy (up to 20% of patients)
- Lipodystrophy (fat redistribution)
- Hyperlactatemia and, rarely, fatal lactic acidosis
- Gastrointestinal upset (nausea, vomiting)
These toxicities prompted the World Health Organization to re‑recommend stavudine only when newer drugs are unavailable or unaffordable.
Criteria for Comparing HIV NRTIs
When you line up stavudine against its peers, most clinicians evaluate five pillars:
- Efficacy - Ability to suppress viral load (measured as % of patients achieving <1000copies/ml).
- Resistance barrier - How quickly the virus can develop mutations that render the drug ineffective.
- Toxicity - Frequency and severity of side effects.
- Dosing convenience - Once daily vs twice daily, pill burden.
- Cost & availability - Price per month in the UK and global procurement programs.
Head‑to‑Head Comparison Table
| Drug (Brand) | Mechanism | Dosing Frequency | Common Side Effects | Resistance Profile | Average UK Monthly Cost | Typical Clinical Use |
|---|---|---|---|---|---|---|
| Zerit (Stavudine) | Thymidine analogue | Twice daily | Neuropathy, lipodystrophy, lactic acidosis | Low barrier - M184V, K65R quickly emerge | £35 | Resource‑limited settings |
| Epivir (Lamivudine) | Cytidine analogue | Once daily | Minimal; rare nausea | High barrier - M184V reduces susceptibility but also reduces viral fitness | £48 | First‑line backbone with tenofovir |
| Viread (Tenofovir disoproxil fumarate) | Adenine analogue | Once daily | Renal tubular dysfunction, bone mineral loss | Moderate - K65R can develop | £65 | First‑line in most guidelines |
| Ziagen (Abacavir) | Guanine analogue | Twice daily | Hypersensitivity (HLA‑B*57:01), mild nausea | High - M184V reduces activity; K65R rare | £70 | Used when renal issues preclude tenofovir |
| Retrovir (Zidovudine) | Thymidine analogue (older) | Twice daily | Anemia, neutropenia, GI upset | Low - K70R, thymidine‑associated mutations | £55 | Historical backbone; limited use today |
| Truvada (Emtricitabine/ Tenofovir) | Combined cytidine + adenine analogue | Once daily (fixed‑dose) | Renal, bone effects (from tenofovir) | High - dual barrier | £90 | Standard first‑line in most high‑income countries |
Deep Dive Into the Alternatives
Lamivudine (Epivir)
Lamivudine’s safety record is impressive. Because it has minimal mitochondrial toxicity, patients rarely report neuropathy or lipoatrophy. Its main drawback is that the M184V mutation, while reducing drug potency, also makes the virus less fit, a paradox that clinicians sometimes exploit.
Tenofovir Disoproxil Fumarate (Viread)
Tenofovir is the workhorse of modern ART. Once‑daily dosing improves adherence, and its viral suppression rates exceed 90% in treatment‑naïve patients. Kidney monitoring is essential, especially for older adults or those on nephrotoxic co‑medications.
Abacavir (Ziagen)
Abacavir shines for patients with pre‑existing renal disease, but the drug carries a risk of a serious hypersensitivity reaction that occurs almost exclusively in individuals with the HLA‑B*57:01 allele. Genetic screening before prescribing is now standard in the UK.
Zidovudine (Retrovir)
One of the first NRTIs, zidovudine can cause bone‑marrow suppression, making it unsuitable for patients with anemia or those on chemotherapy. It is still used in some mother‑to‑child transmission prevention protocols.
Emtricitabine/Tenofovir (Truvada)
The fixed‑dose combination simplifies regimens and offers a high genetic barrier. It’s the backbone of many pre‑exposure prophylaxis (PrEP) programs, but the same renal concerns as tenofovir apply.

Choosing the Right NRTI for You
Below is a quick decision flow you can run through with your clinician:
- If you have easy access to tenofovir and your kidneys are healthy → go with tenofovir (or Truvada) for once‑daily convenience.
- If you have renal impairment or are pregnant → consider lamivudine or abacavir (after HLA‑B*57:01 testing).
- If cost is the dominant factor and you’re in a low‑resource setting → stavudine may still be the most affordable option, but you’ll need close monitoring for neuropathy.
- If you’ve already experienced lipodystrophy on stavudine → switching to lamivudine or tenofovir can often reverse the changes over months.
Remember, every switch should be paired with a fresh viral load test after 4-12 weeks to confirm continued suppression.
Practical Tips for Patients Switching from Zerit
- Schedule a baseline blood work panel (CBC, renal function, fasting lipids).
- Ask about potential drug‑drug interactions - many NRTIs combine with protease inhibitors or integrase inhibitors.
- Set a reminder for the new dosing schedule; even a once‑daily pill can be missed if you’re used to twice‑daily.
- Track any new symptoms in a diary - especially tingling in your feet or sudden weight loss.
- Keep your pharmacy informed; some newer NRTIs require special handling or prior authorisation.
Frequently Asked Questions
Is stavudine still prescribed in the UK?
Stavudine is rarely used in the UK’s NHS formulary today because newer NRTIs offer better safety. It may still be prescribed in specialist clinics for patients who cannot tolerate alternatives, but this is exceptional.
How does the effectiveness of Zerit compare to tenofovir?
In head‑to‑head trials, tenofovir achieved viral suppression in about 92% of treatment‑naïve patients, whereas stavudine reached roughly 78%. The difference is largely due to tenofovir’s higher potency and better tolerability, which improves adherence.
Can I switch from Zerit to lamivudine without a treatment interruption?
Yes, most clinicians make a direct substitution while maintaining the same companion drugs. However, a baseline viral load test and a follow‑up at week 8 are recommended to ensure the new regimen stays effective.
What monitoring is needed after switching off stavudine?
Key labs include CD4 count, HIV RNA, fasting lipids, and renal function. If neuropathy was present, a neurologist assessment can track recovery. Side‑effect check‑ins every month for the first three months help catch any new issues early.
Is there a cost advantage to staying on Zerit?
In the UK, stavudine’s generic price hovers around £35 per month, cheaper than tenofovir (£65) or Truvada (£90). However, the hidden cost of managing side effects can outweigh the drug price, especially in high‑income settings where medical visits are billed separately.
Whether you stick with Zerit or transition to a newer NRTI, the goal stays the same: keep the virus suppressed, protect your overall health, and maintain a quality of life that lets you focus on the things you love.




Elise Smit
October 5, 2025 AT 17:00When you’re managing a regimen that includes Zerit, keep an eye on the mitochondrial side‑effects. Peripheral neuropathy often shows up as tingling in the hands or feet, so regular neurological exams are a must. Lipodystrophy can alter body fat distribution, which may affect patients’ self‑image and adherence. If you notice hyperlactatemia, check lactate levels promptly; early detection can prevent serious complications. Switching to tenofovir or emtricitabine is usually smoother because of the lower toxicity profile, but always verify viral load before making changes.
Sen Đá
October 7, 2025 AT 06:12While the suggestions are sound, the underlying premise that Zerit remains viable in any setting is fundamentally flawed. The drug’s toxicity exceeds acceptable thresholds even in low‑resource environments, and the cost savings are marginal when factoring in the management of adverse events. Moreover, the data cited in the original comparison tool fails to account for recent resistance patterns. A more rigorous, evidence‑based approach would discard stavudine altogether in favor of newer agents with proven safety margins.
Tatiana Akimova
October 8, 2025 AT 19:24Yo, if you’re still on Zerit, you’ve earned major points for toughness, but let’s be real-you deserve a smoother ride. The once‑daily pills like tenofovir not only cut down pill fatigue but also spare you the nerve pain that can ruin a marathon of life. Think of it as upgrading from a flip‑phone to a smartphone-same job, way cooler features. Talk to your provider about a switch, keep that viral load locked down, and you’ll feel the difference in no time.
Calandra Harris
October 10, 2025 AT 08:36Stavudine belongs in the museum, not the pharmacy shelf.
Dan Burbank
October 11, 2025 AT 21:48When you examine the history of antiretroviral therapy, stavudine occupies a peculiar niche as both a breakthrough and a cautionary tale. In the mid‑1990s, its introduction marked a dramatic reduction in AIDS‑related mortality, providing hope to millions across the globe. Yet, the very mechanism that conferred its antiviral potency-mimicking natural nucleosides-also paved the way for mitochondrial interference. This off‑target effect manifested clinically as peripheral neuropathy, a condition that can progress from mild tingling to debilitating pain. Lipodystrophy, another hallmark of stavudine, reshapes body fat distribution, leading to a distinctive “buffalo hump” and facial wasting that can be socially stigmatizing. Hyperlactatemia, though less common, carries the risk of fatal lactic acidosis if unrecognized. The cumulative burden of these toxicities prompted international guidelines to demote stavudine to a last‑line option, especially where newer agents are affordable. Modern alternatives such as tenofovir, emtricitabine, and lamivudine boast efficacy rates upward of 90 % while maintaining toxicity profiles below 15 %. Their once‑daily dosing simplifies adherence, a critical factor in long‑term viral suppression. From an economic perspective, the low acquisition cost of stavudine is offset by the expenses associated with managing its complications. Hospitalizations for neuropathy, diagnostic testing for lactate levels, and the psychosocial impact of body‑image changes can erode any initial savings. Furthermore, resistance patterns have evolved; patients previously on stavudine may harbor mutations that limit the effectiveness of subsequent regimens. Clinicians must therefore weigh the historical legacy of stavudine against the contemporary standards of care, prioritizing patient quality of life. Ultimately, the decision to continue or discontinue stavudine should be individualized, taking into account resource constraints, comorbidities, and patient preferences. In most settings, however, the balance tips decisively toward newer, safer NRTIs that uphold both virologic control and tolerability.
Mina Berens
October 13, 2025 AT 11:00Just read through the comparison tool-pretty handy! 😊 It’s wild how much the dosing schedule can affect daily life. If you’re juggling work or school, once‑daily meds are a game‑changer. Also, keeping an eye on side‑effects early can save a lot of hassle later.
Ismaeel Ishaaq
October 15, 2025 AT 00:12The vivid picture you painted about stavudine’s rise and fall is spot‑on, and it reminds us that medicine is a living, breathing art form. While the drug’s legacy is dotted with challenges, it also taught us invaluable lessons about mitochondrial safety. Today’s arsenal, bursting with bright‑colored pills, reflects that hard‑won wisdom. Let’s celebrate the progress and keep pushing for regimens that sparkle with efficacy and kindness.
Jesse Goodman
October 16, 2025 AT 13:24Life is short; choose the pill that lets you live longer. 🧠
Antara Kumar
October 18, 2025 AT 02:36While the historical analysis is thorough, it overlooks the fact that many low‑income countries still depend on stavudine due to patent restrictions on newer drugs. Dismissing it outright may ignore the realpolitik of drug distribution.
John Barton
October 19, 2025 AT 15:48Oh sure, let’s all drop our beloved Zerit like a hot potato just because it’s “old”. Who needs a cheap, reliable backbone when we can have fancy pills that cost a fortune?
Achint Patel
October 21, 2025 AT 05:00Maybe the sarcasm masks a deeper truth: when cost barriers crumble, patients can finally access therapies that don’t gnaw at their nerves. It’s a subtle reminder that affordability and safety must walk hand‑in‑hand.
Lilly Merrill
October 22, 2025 AT 18:12It’s fascinating how different regions approach HIV treatment based on both economics and cultural attitudes toward medication. In some communities, the stigma around side‑effects can be as powerful as the side‑effects themselves. Sharing clear, culturally sensitive information helps people make informed choices without fear.
Charlie Martin
October 24, 2025 AT 07:24True, but the data also shows that patients who receive proper counseling about potential toxicities are more likely to stay adherent, regardless of cultural background.
Danielle Watson
October 25, 2025 AT 20:36the comparison tool is a good start but it could use more real‑world case studies it feels a bit too textbook
Kimberly :)
October 27, 2025 AT 08:48Even though the tool highlights tenofovir and emtricitabine as preferred, we shouldn’t discount the strategic role of stavudine in certain treatment‑naïve populations. Its ultra‑low price can be a decisive factor where funding is limited, and some clinicians report acceptable tolerance when dosing is carefully adjusted. However, the high toxicity numbers - especially neuropathy rates approaching one in five - mean that vigilant monitoring is non‑negotiable. In my view, stavudine should be positioned as a contingency option, not a default first‑line regimen. The nuanced approach respects both fiscal realities and patient safety 😊
Sebastian Miles
October 28, 2025 AT 22:00Monitoring trough levels and renal function can flag early toxicity, letting clinicians adjust doses before severe adverse events manifest.
Harshal Sanghavi
October 30, 2025 AT 11:12Sure, let’s pretend cost never matters-because we all have unlimited insurance, right?
Theunis Oliphant
November 1, 2025 AT 00:24The discourse suffers from a lamentable lack of epistemic rigor; invoking “acceptable tolerance” without robust meta‑analysis is intellectually irresponsible. One must demand randomized controlled data before elevating such a claim to any semblance of credibility.
India Digerida Para Occidente
November 2, 2025 AT 13:36We can bridge the gap by encouraging collaborative research that evaluates stavudine’s cost‑effectiveness alongside newer agents in resource‑constrained settings. By pooling data, stakeholders can formulate guidelines that are both humane and fiscally sound.
Andrew Stevenson
November 4, 2025 AT 02:48Great points all around-especially the call for shared datasets. In practice, implementing a standardized monitoring protocol across clinics can harmonize safety outcomes. Leveraging existing pharmacovigilance networks will streamline adverse event reporting. Moreover, health economists can model long‑term cost offsets when switching from stavudine to newer NRTIs, factoring in reduced hospital admissions. Training modules for clinicians in low‑resource areas can emphasize dose optimization and early neuropathy detection. Patient education campaigns, delivered in local languages, empower individuals to report symptoms promptly. Ultimately, a multidisciplinary strategy that aligns clinical efficacy, safety, and affordability will elevate HIV care worldwide.