Most people assume that if two pills have the same active ingredient, they work the same way. That’s mostly true - but not always. Behind the scenes, the inactive ingredients in generic medications can cause real problems when taken together. These are the fillers, dyes, preservatives, and binders that don’t treat your condition but help the pill hold its shape, dissolve properly, or taste less awful. The problem? When you’re on multiple generics, those harmless-seeming additives can pile up - and trigger reactions you never saw coming.
Why Inactive Ingredients Matter More Than You Think
The FDA says inactive ingredients don’t affect how a drug works. But that’s not the whole story. A 2020 study found that someone taking ten prescription meds ingests nearly 3 grams of these additives every day. That’s like swallowing a teaspoon of chemicals you didn’t ask for. Most of the time, it’s fine. But for people with sensitivities, allergies, or digestive issues, even small amounts can cause trouble. Take lactose, for example. About 65% of the global population has trouble digesting it. That doesn’t mean you’re lactose intolerant like someone who breaks out in hives after cheese. It means your gut might not handle more than 1-2 grams of lactose at a time. Now imagine you’re on three different generic blood pressure pills, each containing 75 mg of lactose. That’s 225 mg per dose - and if you take them three times a day, you’re hitting 675 mg. For some, that’s enough to cause bloating, cramps, or diarrhea. And since the label won’t say “contains lactose” in bold, you won’t know why you feel off. Same goes for propylene glycol. It’s in nearly half of all liquid generics - think cough syrups, liquid antibiotics, even some heart meds. It’s safe for most people. But in high doses, especially when combined with other meds containing it, it can cause nausea, dizziness, or even kidney stress in older adults. And because different manufacturers use different combinations, two “identical” generic pills might have totally different excipient profiles.Generic Pills Aren’t All the Same - Even When They Should Be
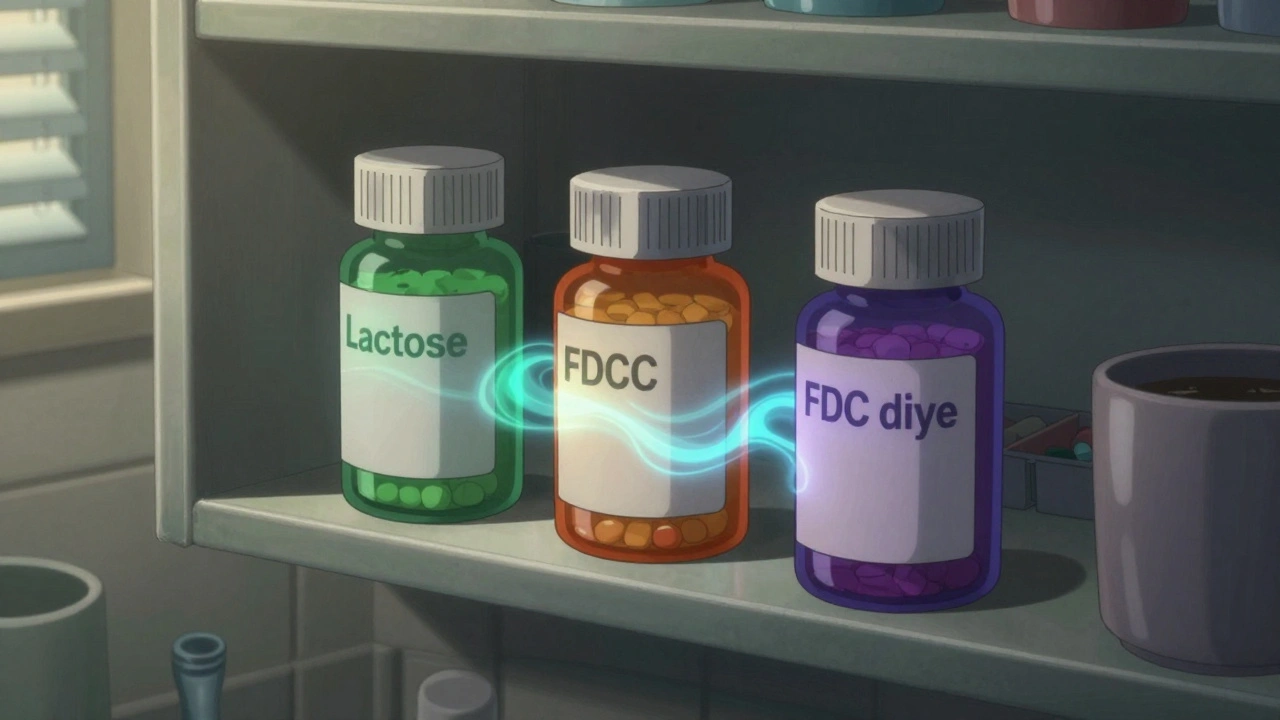
Here’s the kicker: two generic versions of the same drug - say, levothyroxine for thyroid issues - can have up to 27 different combinations of inactive ingredients. That’s not a typo. The FDA’s own database lists that many variations. One version might use corn starch and titanium dioxide. Another might use lactose, magnesium stearate, and FD&C Yellow No. 5. The active ingredient? Identical. The effect? Sometimes not. A 2021 study showed that certain generic antiepileptic drugs had 15-20% lower peak blood levels than their brand-name counterparts - not because the active ingredient was weaker, but because the excipients changed how fast it dissolved in the stomach. For drugs with a narrow therapeutic window - like digoxin, warfarin, or lithium - that kind of variation can mean the difference between control and crisis. Brand-name drugs tend to stick with the same excipients across batches. Generics? Every manufacturer picks what’s cheapest, easiest to source, or easiest to patent around. So if you switch from one generic to another - even if your doctor didn’t change your prescription - you could be getting a completely different chemical mix.Who’s at Risk? It’s Not Just People with Allergies
You might think this only affects people with known allergies. But it’s more common than that. - Lactose-sensitive patients: Even if you’ve never had a problem with milk, you might react to the cumulative dose of lactose across three or four meds. Symptoms: gas, diarrhea, abdominal pain. - Asthmatics: Bisulfites (used as preservatives in some injectables and inhalers) can trigger bronchospasms in 5-10% of asthmatics. If you’re on multiple meds with bisulfites, your risk climbs. - Children and elderly: Their bodies process additives slower. A dye like tartrazine (Yellow No. 5), which affects about 4% of people, can cause hyperactivity in kids or confusion in older adults. - People on five or more meds: The average Medicare beneficiary takes 4.8 prescriptions daily. More meds = more excipients = higher chance of overlap. A 2022 survey found that 23% of community pharmacists see at least one patient per month with unexplained symptoms tied to excipient combinations. One Reddit user, u/MedSafetyWatcher, described how switching to three different generic antidepressants triggered severe GI distress - even though each one was fine alone. Only after digging into the ingredient lists did he realize all three contained lactose. He switched to lactose-free versions and felt better in days.What Pharmacists Are Doing About It
Forward-thinking pharmacists are starting to track this. The American Pharmacists Association now recommends reviewing all inactive ingredients when a patient is on multiple generics - especially if they have unexplained side effects. Here’s how they do it:- Use the FDA’s Inactive Ingredient Database or DailyMed to look up each medication’s full ingredient list by NDC code.
- Identify common problem excipients: lactose, propylene glycol, dyes, bisulfites, gluten (yes, some pills use wheat starch), and alcohol.
- Add up daily exposure. For lactose: under 12 grams is generally safe, but sensitive people react to as little as 1-2 grams.
- Compare with known thresholds. For example, one 100mg lactose tablet = 0.1g. Three a day = 0.3g. If you’re also on a liquid antibiotic with 0.5g of lactose per dose, you’re at 0.8g - enough for some.
- Find alternatives. Not all generics are the same. There’s usually a lactose-free version of metformin, for instance. Or a dye-free version of ibuprofen.
The Bigger Picture: Why This Isn’t Going Away
In 2023, 89% of U.S. prescriptions were filled with generics. That number is only rising. The global generic drug market is projected to hit $234 billion by 2027. More generics = more variation = more chances for hidden interactions. Regulators are starting to pay attention. The FDA launched its Inactive Ingredient Transparency Initiative in January 2024, requiring full digital labeling of all excipients by December 2025. The European Medicines Agency already requires manufacturers to justify using excipients that affect more than 0.1% of the population. New tools are helping too. MedCheck AI, released in late 2023, scans prescriptions and flags potential excipient overlaps with 89.7% accuracy. It’s not perfect - but it’s a start. Still, the system isn’t built for this. Doctors don’t learn about excipients in med school. Pharmacists are overworked. Patients don’t know to ask. And insurance companies? They push for the cheapest generic - regardless of ingredients.
What You Can Do Right Now
You don’t need to be a scientist to protect yourself. Here’s what works:- Ask your pharmacist: “Do these generics have the same fillers?” Show them your list of meds.
- Check the label. Look for “inactive ingredients” or “other ingredients.” Write them down.
- If you’ve had unexplained symptoms - stomach issues, rashes, fatigue, or reduced effectiveness - suspect excipients. Try switching one generic at a time to see if it helps.
- Use apps like Medisafe or MyTherapy to track your meds and their ingredients. Some now include excipient alerts.
- If you’re sensitive to something (lactose, dyes, etc.), ask for “lactose-free” or “dye-free” versions. They exist.
The Bottom Line
Generic drugs save billions. That’s good. But assuming they’re all identical is dangerous. Inactive ingredients aren’t just filler - they’re active players in how your body reacts to treatment. When you stack them up, the risks multiply. This isn’t about blaming generics. It’s about awareness. If you’re on multiple meds - especially older, sicker, or more sensitive - don’t assume safety just because the name on the bottle matches your prescription. Ask. Check. Switch if needed. Your body might thank you.Can inactive ingredients in generic drugs really cause side effects?
Yes. While inactive ingredients don’t treat your condition, they can trigger reactions in sensitive people. Common examples include lactose causing bloating in intolerant individuals, propylene glycol leading to nausea in high doses, and dyes like tartrazine triggering skin rashes or hyperactivity. Cumulative exposure from multiple generics can push someone past their tolerance threshold, even if each individual medication was fine alone.
How do I find out what’s in my generic medication?
Check the package insert or the FDA’s Inactive Ingredient Database using the drug’s NDC code. You can also ask your pharmacist - they have access to detailed ingredient lists. Look for the section labeled “Inactive Ingredients” or “Other Ingredients.” Many manufacturers don’t list everything on the bottle, so don’t rely on the outer packaging alone.
Are brand-name drugs safer than generics when it comes to inactive ingredients?
Not necessarily safer, but often more consistent. Brand-name drugs usually stick to the same excipients across batches, reducing variability. Generics can change ingredients between manufacturers - or even between batches from the same maker. That’s why switching from one generic to another - even for the same drug - can sometimes cause unexpected reactions.
What should I do if I think my meds are causing reactions due to inactive ingredients?
Don’t stop taking your meds. Talk to your pharmacist first. They can help identify if any of your drugs share problematic excipients. Then, ask if there’s a lactose-free, dye-free, or alcohol-free alternative available. Often, multiple generic versions exist - you just need to request the right one. Many patients report symptom relief within days of switching to a compatible formulation.
Are there any tools to check for excipient interactions?
Yes. Tools like MedCheck AI (released in 2023) scan your prescription list and flag potential excipient overlaps with nearly 90% accuracy. The FDA’s Inactive Ingredient Database and DailyMed are free public resources you can use to look up ingredients by drug name or NDC code. Some pharmacy apps, like Medisafe, now include excipient alerts as part of their tracking features.

Jade Hovet
December 13, 2025 AT 05:07OMG I had no idea!! I’ve been on 5 generics for years and my stomach’s been killing me?? I just thought I was eating wrong 😭 switched my metformin to lactose-free last week and I’m already not bloating like a balloon. Pharmacist was like ‘yeah, that’s a thing’ 🤦♀️ why isn’t this on the bottle??
nithin Kuntumadugu
December 14, 2025 AT 08:13LMAO so now the FDA is gonna ‘regulate’ fillers?? 😂 Meanwhile Big Pharma is laughing all the way to the bank with their ‘special’ lactose-free versions that cost 3x more. This is just another way to sell you the same drug with a fancy label. Wake up people - it’s all about profit, not your gut.
Ronan Lansbury
December 15, 2025 AT 20:39How quaint. You’re all treating this like some novel discovery. The pharmaceutical industry has been obscuring excipient profiles since the 1980s. The FDA’s database? A joke. It’s incomplete, inconsistently updated, and deliberately vague. I’ve personally cross-referenced 117 NDC codes - over 40% didn’t list allergens properly. This isn’t negligence. It’s structural obfuscation. And you’re all just… scrolling.
John Fred
December 17, 2025 AT 09:15Y’all need to start using MedCheck AI - it’s a game-changer. I’m a pharmacist and I’ve been using it for 6 months now. It flags overlaps like a boss. Also, don’t forget to check for alcohol in liquid meds - it’s in more than you think. And yes, some generics use wheat starch. Gluten sneaks in. 😬 Stay informed, stay safe - your body’s not a lab rat.
Harriet Wollaston
December 19, 2025 AT 02:22This is such an important topic and I’m so glad someone brought it up. I have a friend with chronic migraines who switched out all her dyes and saw a 70% drop in flare-ups. It’s not magic - it’s just chemistry. We don’t talk enough about what’s *in* the pill, not just what it’s *for*. You’re not crazy if you feel weird after a med switch. It’s probably not you - it’s the filler.
nina nakamura
December 19, 2025 AT 15:32Stop whining. If you can’t handle a little lactose or dye you’re weak. People in developing countries take pills with chalk and sawdust and survive. Your sensitivity is a luxury. The system works fine - you just don’t like being told what to do. Take your meds and stop blaming the filler.
Constantine Vigderman
December 21, 2025 AT 10:46Wait so if I take three different generics for blood pressure and they all have propylene glycol… I’m basically drinking a tiny bit of antifreeze every day?? 😳 That’s wild. I’m checking my meds right now. Also - anyone know if the CVS brand of lisinopril is lactose-free? I’m dying to know.
Cole Newman
December 22, 2025 AT 16:57Why are you all so obsessed with this? Just take the damn pill. If you’re that sensitive maybe you should be on brand name. It’s not rocket science. You want control? Pay more. Simple. Stop making it a conspiracy.
sharon soila
December 23, 2025 AT 00:57Let’s remember something beautiful: your body is trying to tell you something. When you feel off after a med change - it’s not ‘all in your head.’ It’s science. And science says: listen. Ask. Check. Switch. You are not a burden. You are a person with a right to feel well. And that’s not too much to ask.
Hamza Laassili
December 24, 2025 AT 02:53Y’all are gonna love this: the FDA lets companies use ‘other ingredients’ to hide crap. I called 3 manufacturers. Two said ‘we don’t list everything’ because ‘it’s proprietary.’ PROPRIETARY?! It’s in my BODY. This is why America’s healthcare is a joke. We’re being fed poison and told to be grateful. #MedScam