80-125 Rule: What It Means for Generic Drug Approval and FDA Compliance
When you pick up a generic pill, you expect it to work just like the brand-name version. That’s not luck—it’s the 80-125 rule, a bioequivalence standard used by the FDA to ensure generic drugs deliver the same therapeutic effect as their brand-name counterparts. Also known as the 80% to 125% bioequivalence range, this rule is the quiet backbone of every generic medication you take. If a generic drug’s active ingredient enters your bloodstream at a rate and amount that falls between 80% and 125% of the brand-name drug, the FDA considers them therapeutically equal. No guesswork. No exceptions.
This rule doesn’t just apply to one drug—it’s used across hundreds of generics, from blood pressure pills to antibiotics. It’s why a $5 version of lisinopril works the same as the $50 brand. But it’s not just about matching numbers. The bioequivalence, the measurable comparison of how quickly and completely a drug is absorbed into the bloodstream is tested in real people under strict conditions. These studies track blood levels over time, and the data must pass rigorous statistical checks. If the results drift outside that 80-125 window, the FDA rejects the application. No second chances.
And it’s not just the drug itself. The pharmaceutical manufacturing, the entire process of producing the drug, from raw ingredients to final packaging must follow CGMP rules. A generic drug might have the right chemistry, but if it’s made in a dirty facility or with inconsistent batches, it can still fail. That’s why the FDA inspects factories—because the 80-125 rule only works if the product you get every time is exactly the same. One batch might pass bioequivalence, but if the next batch doesn’t, the whole line gets shut down.
What you might not realize is that this rule protects you from dangerous variations. A drug that’s 70% as effective could mean your blood pressure stays high. One that’s 150% as potent might cause overdose symptoms. The 80-125 range isn’t arbitrary—it’s based on decades of clinical data and real-world outcomes. It’s the reason you can trust a generic without asking your doctor for the brand name every time.
When you see a generic drug on the shelf, you’re seeing the result of this rule in action. It’s why millions of people save money without sacrificing safety. But it’s also why the FDA keeps a close eye on manufacturers. Violations in production—like data falsification or poor quality control—can undermine the entire system. That’s why posts on CGMP compliance, FDA inspections, and manufacturing deficiencies are so critical. They’re the behind-the-scenes checks that keep the 80-125 rule meaningful.
Below, you’ll find real-world examples of how this rule plays out—from the labs where bioequivalence is tested to the factories that must prove they can repeat results, batch after batch. You’ll see what happens when things go wrong, how regulators respond, and why your next generic prescription is safer than you think.
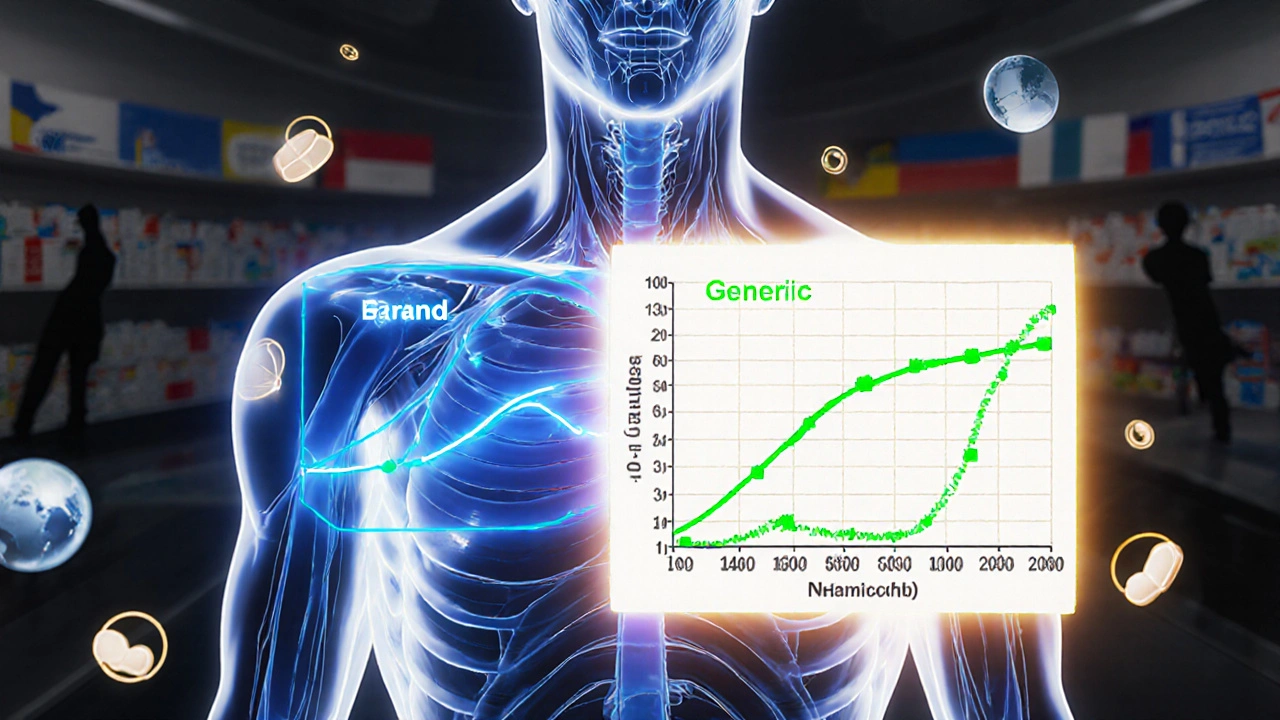
The 80-125% Rule: Understanding Bioequivalence Confidence Intervals in Generic Drugs
The 80-125% rule ensures generic drugs are absorbed similarly to brand-name versions. It's based on pharmacokinetic data, not active ingredient amounts, and is used globally to approve safe, affordable generics.
Detail