Bioequivalence Explained: What It Means for Generic Drugs and Your Health
When you switch from a brand-name pill to a cheaper generic, you’re counting on one thing: bioequivalence, the scientific proof that two drug versions deliver the same amount of active ingredient at the same rate, so they work the same way in your body. Also known as therapeutic equivalence, it’s the reason your pharmacist can legally swap out expensive pills for ones that cost a fraction of the price. Without bioequivalence, generics wouldn’t be trusted—or even allowed—by regulators like the FDA or EMA. It’s not just about cost. It’s about safety, consistency, and knowing your treatment won’t suddenly stop working because you picked the lower-priced option.
Bioequivalence isn’t guessed at—it’s measured. Companies run strict tests using healthy volunteers, tracking how fast and how much of the drug enters the bloodstream. The results must fall within a narrow range: 80% to 125% of the brand-name drug’s performance. That’s not a big gap. It’s tight enough to ensure your blood pressure stays controlled, your seizures stay away, or your cholesterol stays down—no matter which version you take. This applies to everything from generic drugs like atorvastatin or sildenafil to complex formulations like extended-release pills or patches. If a generic fails this test, it doesn’t hit the market.
But bioequivalence doesn’t mean every generic is identical in every way. Fillers, coatings, and inactive ingredients can differ. That’s why some people notice a change in side effects or how quickly a pill works—especially with drugs that have a narrow therapeutic window, like warfarin or levothyroxine. That’s not bioequivalence failing. It’s your body reacting to something else in the pill. Still, regulators require proof that these differences don’t change how the drug behaves in your system. And when they do? That’s when you hear about recalls or warnings.
You’ll find bioequivalence mentioned in posts about generic medication, the legally approved, lower-cost versions of brand-name drugs that must meet the same bioequivalence standards, like buying cheap Lipitor or Viagra online. It’s why some online pharmacies get flagged—not because they sell generics, but because they sell ones that never proved they’re equivalent. The same goes for switching from Prometrium to a generic progesterone, or comparing Aceon to lisinopril. If the drug’s bioequivalence hasn’t been verified, you’re gambling with your health.
And it’s not just about pills. Bioequivalence rules apply to inhalers, injections, even topical creams. If a generic version of a skin cream doesn’t release the drug at the same rate as the brand, it won’t work the same. That’s why you can’t just assume all generic versions are equal. You need to trust the system—and that system only works when manufacturers prove bioequivalence with real data, not promises.
Below, you’ll find real-world examples of how bioequivalence affects your choices. Whether you’re comparing HIV meds, checking out generic sildenafil, or wondering why your blood pressure pill changed, these posts cut through the noise. They show you which switches are safe, which ones need caution, and how to spot when something doesn’t add up. No fluff. Just what you need to know to make smart decisions about your meds.

Therapeutic Equivalence: What It Really Means for Patient Safety
Therapeutic equivalence ensures generic drugs work just like brand-name ones, keeping patients safe while cutting costs. Learn how the FDA verifies this, what the Orange Book codes mean, and why switching generics is usually safe.
Detail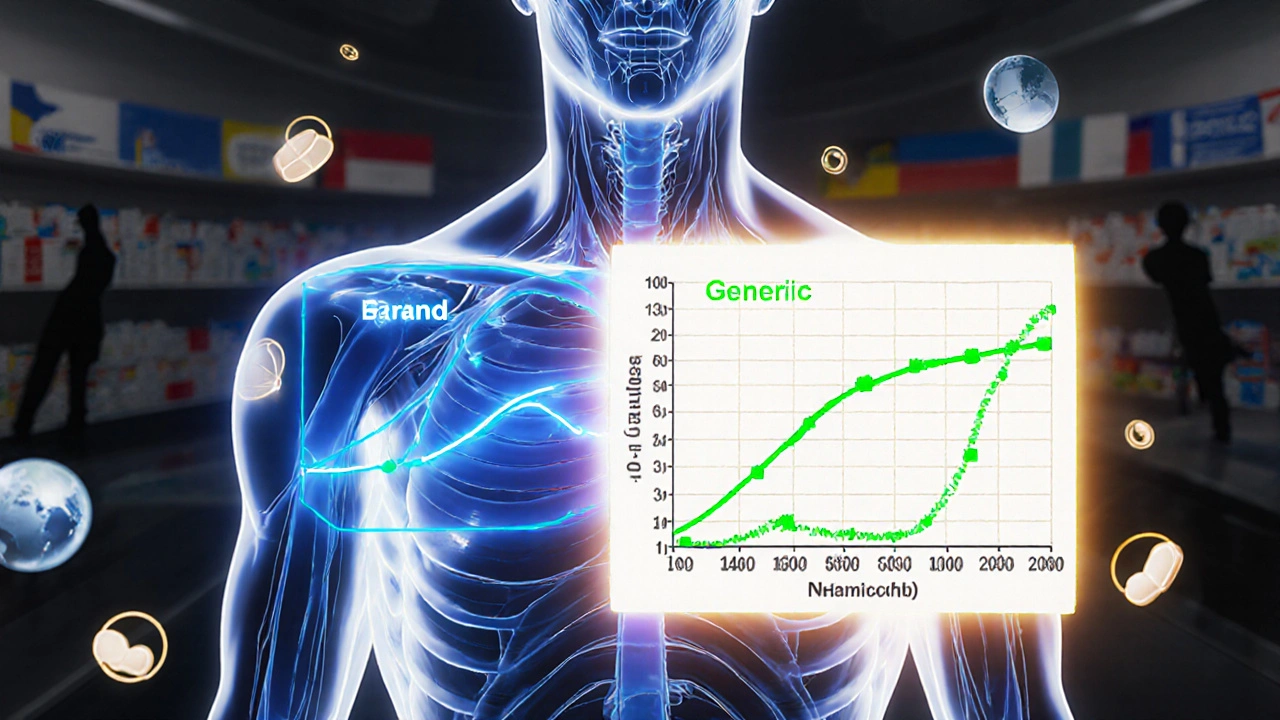
The 80-125% Rule: Understanding Bioequivalence Confidence Intervals in Generic Drugs
The 80-125% rule ensures generic drugs are absorbed similarly to brand-name versions. It's based on pharmacokinetic data, not active ingredient amounts, and is used globally to approve safe, affordable generics.
Detail




