Author: Ryan Gregory - Page 3
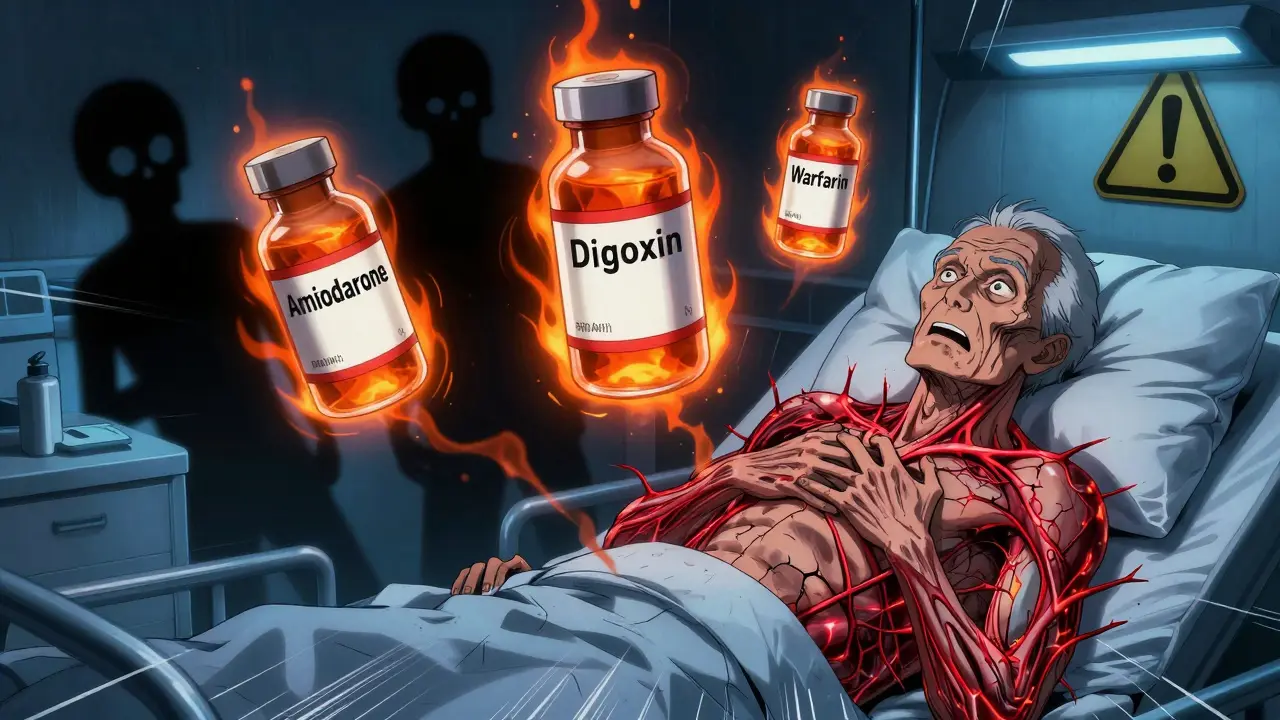
Amiodarone, Digoxin, and Warfarin: The Dangerous Drug Triad You Can't Afford to Miss
Amiodarone, digoxin, and warfarin together can cause deadly drug interactions. Learn how this triad raises the risk of digoxin toxicity and life-threatening bleeding - and what you must do to stay safe.
Detail
Bulk Purchasing and Discounts: How Large-Scale Procurement of Generic Medications Lowers Costs
Bulk purchasing generic medications can cut drug costs by 20% or more for clinics and providers. Learn how volume discounts, PBM rebates, and secondary distributors work-and how to start saving today.
Detail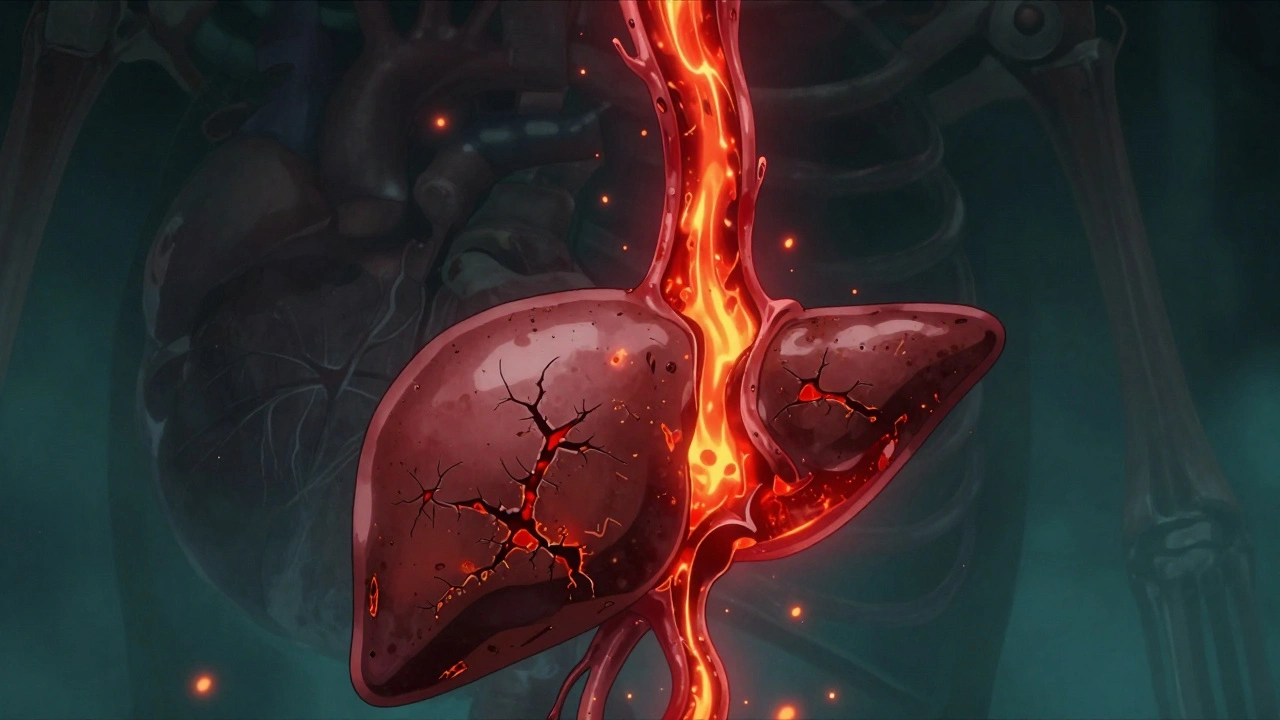
Hemochromatosis: How Iron Overload Damages Your Liver and How Phlebotomy Fixes It
Hemochromatosis is a genetic iron overload disorder that damages the liver, heart, and pancreas. Early diagnosis through blood tests and treatment with phlebotomy can prevent serious complications - if caught before cirrhosis develops.
Detail
How to Use Manufacturer Copay Assistance Cards to Lower Prescription Costs
Manufacturer copay assistance cards can slash your prescription costs-but only if you understand how they work. Learn how to use them, avoid hidden traps like copay accumulators, and plan ahead before your savings run out.
Detail
Irritable Bowel Syndrome: Symptoms, Triggers, and Medication Options
Irritable Bowel Syndrome causes abdominal pain, bloating, and unpredictable bowel changes. Learn the key symptoms, common triggers like FODMAPs and stress, and proven medication options for IBS-D, IBS-C, and mixed types.
Detail
Anticoagulants: Warfarin vs DOACs - Safety, Risks, and What You Need to Know
Warfarin and DOACs both prevent dangerous clots, but DOACs are safer and easier to use for most people. Learn the real differences in bleeding risk, kidney safety, cost, and adherence - and what to ask your doctor.
DetailAnticonvulsants and Birth Control: What You Need to Know About Reduced Effectiveness
Many anticonvulsants like carbamazepine and topiramate reduce birth control effectiveness by speeding up hormone metabolism. This can lead to unintended pregnancy. Learn which seizure meds interfere, which contraceptives still work, and what to do next.
Detail
How to Verify Controlled Substance Quantities and Directions: A Step-by-Step Guide for Pharmacists
Learn the exact steps pharmacists must follow to verify controlled substance quantities and directions to avoid legal penalties, prevent diversion, and ensure patient safety. Includes DEA math, PDMP checks, CDC conversion factors, and real-world pitfalls.
Detail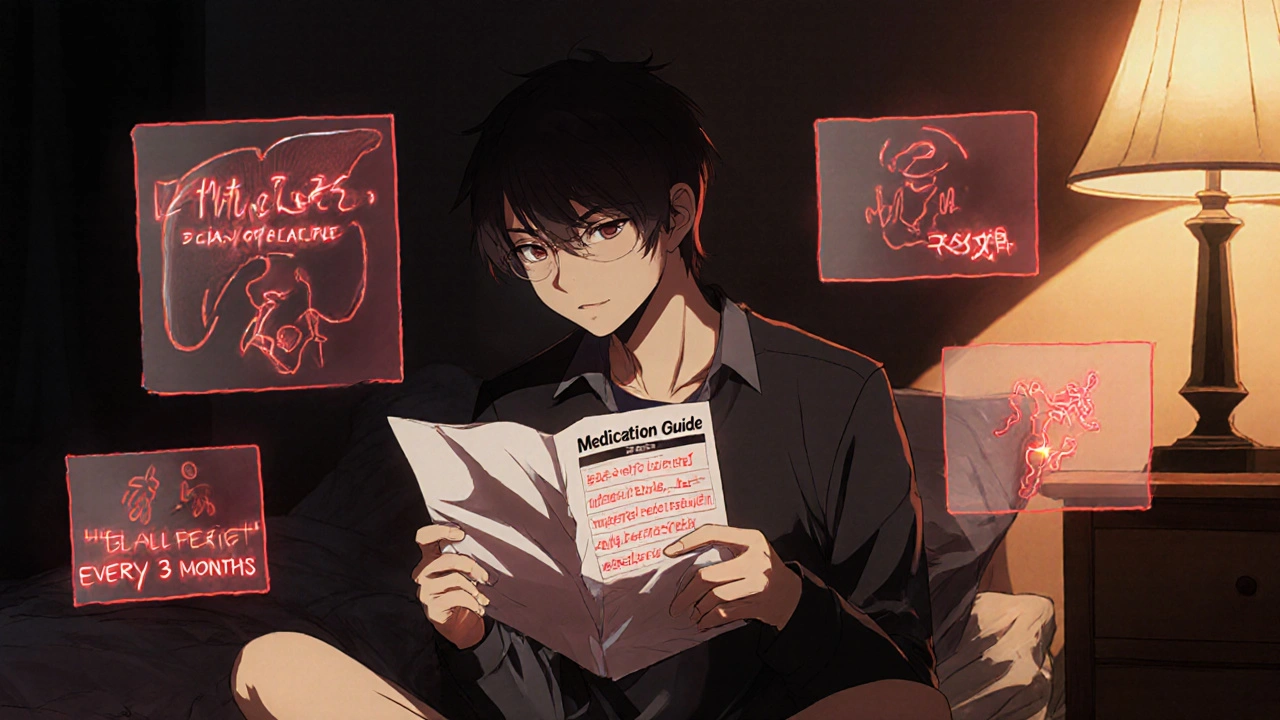
How to Read Medication Guides to Understand Risk and Monitoring Requirements
Learn how to read FDA-required Medication Guides to spot serious drug risks and follow critical monitoring steps. Understand what to look for, how to act, and why skipping this guide could be dangerous.
Detail
Compounding Pharmacies: What to Do When Your Medication Isn't Available
When your medication runs out and isn't coming back, compounding pharmacies offer customized alternatives tailored to your needs-no allergens, perfect doses, or easier forms. Here's how they work and when to use them.
Detail